The nuclear chemistry worksheet with answers provides a comprehensive overview of the field of nuclear chemistry, covering fundamental concepts, reactions, and applications. This worksheet is an invaluable resource for students, educators, and professionals seeking to deepen their understanding of this fascinating and complex subject.
Nuclear chemistry involves the study of the structure, properties, and reactions of atomic nuclei. It explores the fundamental building blocks of matter and the forces that govern their interactions. This worksheet delves into the intricacies of nuclear reactions, including fission and fusion, and their applications in diverse fields such as energy production and medical diagnostics.
1. Introduction to Nuclear Chemistry
Nuclear chemistry is the study of the structure, properties, and reactions of atomic nuclei. It encompasses the study of the fundamental particles that make up the nucleus, the forces that hold them together, and the changes that occur when nuclei undergo reactions.
The nucleus is the central part of an atom, and it contains protons and neutrons. Protons are positively charged particles, while neutrons are neutral. The number of protons in the nucleus determines the element to which the atom belongs. The number of neutrons in the nucleus can vary, giving rise to isotopes of the same element.
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. For example, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon. They all have six protons, but they have different numbers of neutrons (6, 7, and 8, respectively).
Isotopes have different physical and chemical properties. For example, carbon-12 is stable, while carbon-14 is radioactive. Radioactive isotopes decay over time, emitting particles and energy. The decay of radioactive isotopes can be used to date objects and to study geological processes.
2. Nuclear Reactions and Equations: Nuclear Chemistry Worksheet With Answers
Nuclear reactions are processes that involve changes in the structure of atomic nuclei. Nuclear reactions can be either spontaneous or induced. Spontaneous nuclear reactions occur naturally, while induced nuclear reactions are caused by the bombardment of nuclei with particles such as neutrons or protons.
There are many different types of nuclear reactions, including:
- Nuclear fission:In nuclear fission, a heavy nucleus splits into two or more lighter nuclei, releasing a great amount of energy.
- Nuclear fusion:In nuclear fusion, two or more light nuclei combine to form a heavier nucleus, releasing a great amount of energy.
- Nuclear transmutation:In nuclear transmutation, one element is converted into another element by a nuclear reaction.
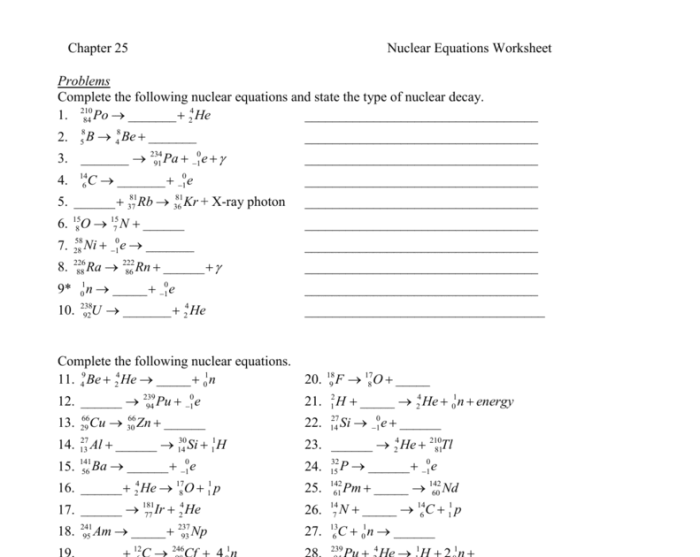
Nuclear reactions can be represented by nuclear equations. Nuclear equations must be balanced, meaning that the total number of protons and neutrons on both sides of the equation must be the same.
Nuclear reactions are governed by the laws of conservation of mass and energy. The total mass and energy of the reactants in a nuclear reaction must be equal to the total mass and energy of the products.
3. Radioactivity and Decay Processes
Radioactivity is the spontaneous decay of atomic nuclei. Radioactive isotopes emit particles and energy as they decay. The type of particle emitted depends on the isotope.
There are three main types of radioactive decay:
- Alpha decay:In alpha decay, an alpha particle (a helium nucleus) is emitted from the nucleus.
- Beta decay:In beta decay, a beta particle (an electron or a positron) is emitted from the nucleus.
- Gamma decay:In gamma decay, a gamma ray (a high-energy photon) is emitted from the nucleus.
The decay of radioactive isotopes can be used to date objects and to study geological processes.
The half-life of a radioactive isotope is the amount of time it takes for half of the atoms in a sample to decay. The half-life of a radioactive isotope is constant and can be used to determine the age of objects.
4. Applications of Nuclear Chemistry
Nuclear chemistry has a wide range of applications in various fields, including:
- Energy production:Nuclear power plants use nuclear fission to generate electricity.
- Medical diagnosis and treatment:Radioactive isotopes are used in medical imaging techniques such as PET scans and in radiation therapy to treat cancer.
- Industrial applications:Radioactive isotopes are used in industrial applications such as material analysis and tracing techniques.
Nuclear chemistry is a rapidly growing field with a wide range of applications. As our understanding of nuclear chemistry continues to grow, we can expect to see even more applications of this technology in the future.
5. Safety and Waste Management in Nuclear Chemistry
Nuclear chemistry is a potentially hazardous field, and it is important to take precautions to ensure the safety of workers and the public.
Radioactive materials must be handled with care to avoid exposure to radiation. Workers who handle radioactive materials must wear protective clothing and equipment.
Radioactive waste must be disposed of properly to avoid environmental contamination. There are a number of different methods for disposing of radioactive waste, including storage in deep geological repositories and transmutation.
Nuclear chemistry is a vital field that has a wide range of applications. However, it is important to use nuclear chemistry safely and responsibly to protect the health and safety of workers and the public.
Detailed FAQs
What is the scope of nuclear chemistry?
Nuclear chemistry encompasses the study of the structure, properties, and reactions of atomic nuclei, including their composition, stability, and interactions.
How are nuclear equations balanced?
Nuclear equations are balanced by ensuring that the total number of protons and neutrons on both sides of the equation remains the same.
What are the different types of radioactive decay?
The primary types of radioactive decay include alpha decay, beta decay, and gamma decay, each involving the emission of different particles or energy.