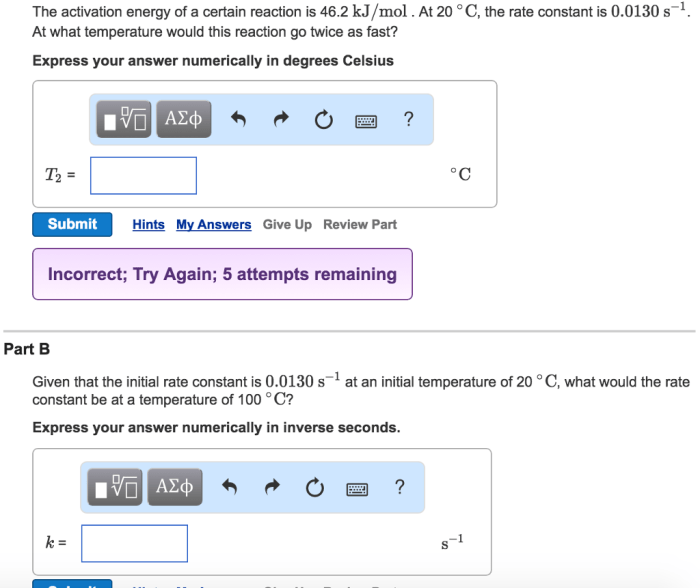
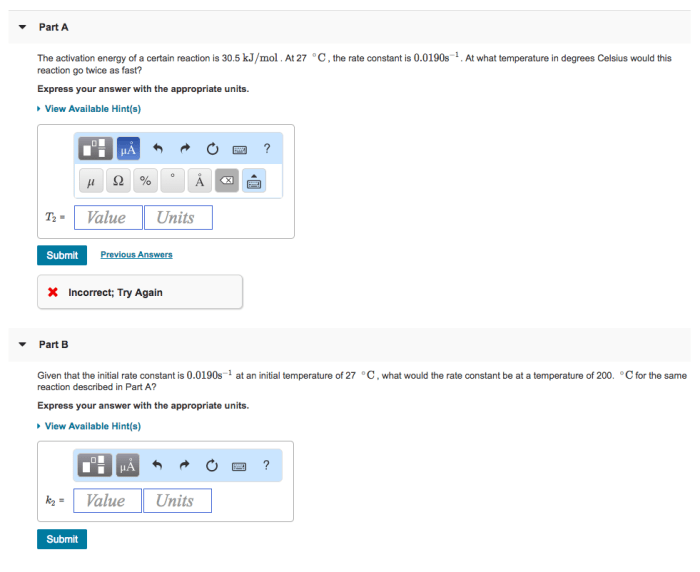
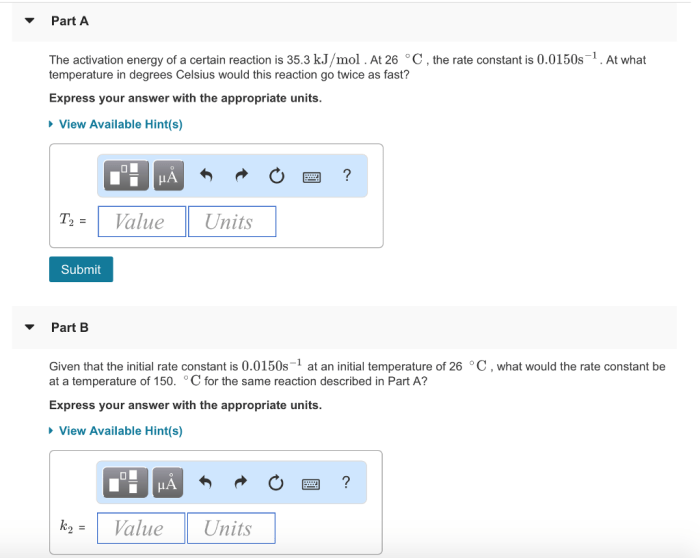
A certain reaction has an activation energy of 54.0 kj/mol – As a certain reaction takes center stage with an activation energy of 54.0 kJ/mol, this opening passage invites readers into a realm of scientific exploration, where the intricacies of chemical transformations are laid bare. Activation energy, a pivotal concept in understanding reaction dynamics, holds the key to unlocking the mysteries of how and why reactions proceed at varying rates.
Join us as we delve into the captivating world of activation energy, examining its profound influence on reaction kinetics and its practical applications across diverse scientific disciplines.
Delving deeper into the topic, we will explore the concept of activation energy, its significance in chemical reactions, and its role in determining reaction rates. We will also investigate the factors that influence activation energy, such as temperature, concentration, and solvent effects.
Furthermore, we will delve into the experimental methods used to measure activation energy and the practical applications of this knowledge in fields such as chemistry, engineering, and medicine.
Understanding Activation Energy
Activation energy is the minimum amount of energy that must be supplied to a system for a chemical reaction to occur. It represents the energy barrier that must be overcome for reactants to transform into products. The significance of activation energy lies in its influence on reaction rates, as reactions with higher activation energies proceed at slower rates.
The Role of Activation Energy in Reaction Rates
The relationship between activation energy and reaction rate is inversely proportional. Reactions with lower activation energies have faster reaction rates, while reactions with higher activation energies have slower reaction rates. Catalysts play a crucial role in reducing activation energy, thereby increasing reaction rates.
Measuring Activation Energy, A certain reaction has an activation energy of 54.0 kj/mol
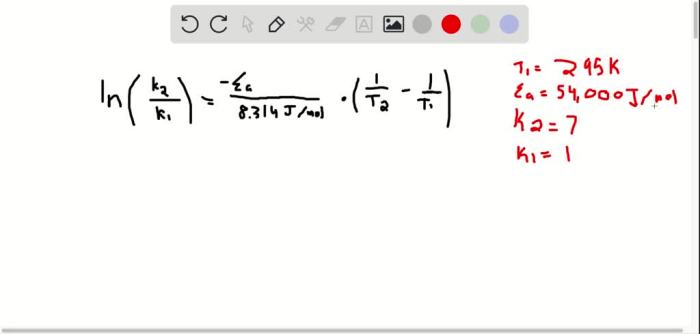
Experimental methods for determining activation energy include the use of the Arrhenius equation, which relates the rate constant of a reaction to the activation energy, temperature, and a pre-exponential factor. The Arrhenius plot, a graphical representation of the Arrhenius equation, is used to calculate activation energy from experimental data.
Factors Influencing Activation Energy
Several factors can influence activation energy, including:
- Temperature:Higher temperatures generally lead to lower activation energies.
- Concentration:Increasing the concentration of reactants can increase activation energy.
- Solvent effects:Solvents can influence activation energy by solvating reactants and transition states.
Applications of Activation Energy: A Certain Reaction Has An Activation Energy Of 54.0 Kj/mol
Activation energy finds applications in various fields:
- Chemistry:Understanding activation energy is essential for optimizing chemical reactions and designing new catalysts.
- Engineering:Activation energy is considered in the design of engines and other devices that involve chemical reactions.
- Medicine:Activation energy plays a role in drug design and understanding enzyme-catalyzed reactions in the body.
FAQ
What is activation energy?
Activation energy is the minimum amount of energy that must be supplied to a system in order for a reaction to occur.
How does activation energy affect reaction rates?
Activation energy has an inverse relationship with reaction rates. The higher the activation energy, the slower the reaction rate.
What factors can influence activation energy?
Factors that can influence activation energy include temperature, concentration, and solvent effects.
How is activation energy measured?
Activation energy can be measured using experimental methods such as the Arrhenius equation.
What are some practical applications of activation energy?
Activation energy has practical applications in fields such as chemistry, engineering, and medicine.
